Insecticide
Beta-cypermethrin
TC & FORMULATION: 95%TC, 50G/L SC, 100G/L SC, 100G/L EC, 5%WP
Structure Formula: 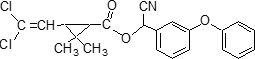
NOMENCLATURE
Common name beta-cypermethrin (BSI, draft E-ISO)
IUPAC name Roth: A reaction mixture comprising two enantiomeric pairs in ratio c. 2:3 (S)-a-cyano-3-phenoxybenzyl (1R)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and
(R)-a-cyano-3-phenoxybenzyl (1S)-cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
with (S)-a-cyano-3-phenoxybenzyl (1R)-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate and
(R)-a-cyano-3-phenoxybenzyl (1S)-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
Chemical Abstracts name cyano(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate; 2 parts of enantiomer pair [(1R)-1a(S*),3a] and [(1S)-1a(R*),3a] with 3 parts of enantiomer pair [(1R)-1a(S*),3b] and [(1S)-1a(R*),3b]
Other names asymethrin*(rejected common name proposal)
CAS RN [52315-07-8] (undefined stereochemistry, also used for cypermethrin); [72204-43-4] ([(1S)-1a(R*),3a] isomer; Roth: (S) (1R)-cis- isomer); [65731-84-2] ([(1R)-1a(S*),3a] isomer; Roth: (R) (1S)-cis- isomer); [83860-31-5] ([(1S)-1a(R*),3b] isomer; Roth: (S) (1R)-trans- isomer); [65732-07-2] ([(1R)-1a(S*),3b] isomer; Roth: (R) (1S)-trans- isomer) EEC no. 276-457-7 (S) (1R)-cis- isomer; 265-898-0 (R) (1S)-cis- isomer; 281-086-9 (S) (1R)-trans- isomer; 265-896-6 (R) (1S)-trans- isomer
PHYSICAL CHEMISTRY
Composition A mixture consisting of two enantiomeric pairs in ratio 2:3: (S) (1R)-cis and (R) (1S)-cis with (S) (1R)-trans and (R) (1S)-trans. Tech. grade beta-cypermethrin contains ³95% (normally >97%) relevant stereoisomers. Mol. wt. 416.3 M.f. C22H19Cl2NO3 Form Tech. forms colourless or pale yellow crystals. M.p. 63.1-69.2 ºC (varies with even a small - 1% - change in isomer ratio) B.p. 286.1?.06 ºC/97.4 kPa V.p. 1.8 ´ 10-4 mPa (20 ºC) KOW logP = 4.7 ?.04 S.g./density 1.336?.0050 (20 ºC) Solubility In water 51.5 (5 ºC), 93.4 (25 ºC), 276.0 (35 ºC) (all in mg/l, pH 7). In isopropanol 11.5, xylene 349.8, dichloromethane 3878, acetone 2102, ethyl acetate 1427, petroleum ether 13.1 (all in mg/ml, 20 ºC). Stability Stable to 150 ºC; to air and sunlight; in neutral and slightly acidic media; epimerised in presence of base, hydrolysed in strongly alkaline media. DT50 (extrapolated) 50 d (pH 3, 5, 6), 40 d (pH 7), 20 d (pH 8), 15 d (pH 9) (all at 25 ºC).
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Non-systemic insecticide with contact and stomach action. Uses It can be used against a wide range of insect pests in public health (e.g. flies, cockroaches, mosquitoes, fleas, lice, bugs) and in veterinary applications (ectoparasitic ticks and mites). In plant protection, it is effective against Coleoptera and Lepidoptera and gives good protection against Orthoptera, Diptera, Hemiptera, and Homoptera. Mainly used in alfalfa, cereals, cotton, grapes, maize, oilseed rape, pome fruit, potatoes, soya beans, sugar beet, tobacco and vegetables. Formulation types CS; EC; ME; SC; UL; gel.
About price and product information, please click Message button.
Open the consulting page, please Writing information, customer manager will reply within 24 hours!
or Contact email:Donna.wen@molotuschem.com
Cell-phone number:17717500809
Tel:0086 21 61203246
Fax:0086 21 61612025
Address:Room 402, No.553, Maotai Road, Changning District, Shanghai.