Insecticide
Abamectin
TC & FORMULATION: 95%TC, 1.8%EC, 3.6%EC
Structure Formula 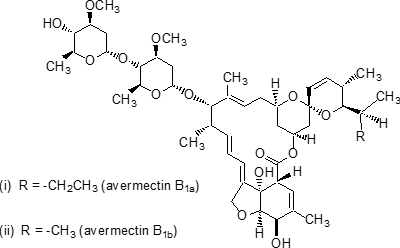
NOMENCLATURE
Common name abamectin (BSI, draft E-ISO, ANSI); abamectine ((f) draft F-ISO)
IUPAC name (10E,14E,16E,22Z)-(1R,4S,5'S,6S,6'R,8R,12S,13S,20R,21R,24S)-6'-[(S)-sec-butyl]-21,24-dihydroxy-5',11,13,22-tetramethyl-2-oxo-3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6-spiro-2'-(5',6'-dihydro-2'H-pyran)-12-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-a-L-arabino-hexopyranosyl)-3-O-methyl-a-L-arabino-hexopyranoside (i) mixture with (10E,14E,16E,22Z)-(1R,4S,5'S,6S,6'R,8R,12S,13S,20R,21R,24S)-21,24-dihydroxy-6'-isopropyl-5',11,13,22-tetramethyl-2-oxo-3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6-spiro-2'-(5',6'-dihydro-2'H-pyran)-12-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-a-L-arabino-hexopyranosyl)-3-O-methyl-a-L-arabino-hexopyranoside (ii) (4:1)
Chemical Abstracts name 5-O-demethylavermectin A1a (i) mixture with 5-O-demethyl-25-de(1-methylpropyl)-25-(1-methylethyl)avermectin A1a (ii) Other names avermectin B1 CAS RN [71751-41-2] (abamectin); [65195-55-3] (i); [65195-56-4] (ii) EEC no. 265-610-3 (avermectin B1a); 265-611-9 (avermectin B1b)
PHYSICAL CHEMISTRY
Composition A mixture containing ³80% avermectin B1a (i) and £20% avermectin B1b (ii). Mol. wt. 873.1 (avermectin B1a); 859.1 (avermectin B1b) M.f. C48H72O14 (avermectin B1a); C47H70O14 (avermectin B1b) Form Colourless to pale yellow crystals. M.p. 161.8-169.4 °C (decomp.) V.p. <3.7 ´ 10-3 mPa (25 °C) KOW logP = 4.4?.3 (pH 7.2, room temperature) Henry 2.7 ´ 10-3 Pa m3 mol-1 (25 °C) S.g./density 1.18 (22 °C) Solubility In water 7-10 mg/l (20 ºC). In toluene 350, acetone 100, isopropanol 70, chloroform 25, ethanol 20, methanol 19.5, n-butanol 10, cyclohexane 6 (all in g/l, 21 ºC). Stability Stable to hydrolysis in aqueous solutions at pH 5, 7, and 9 (25 ºC). Sensitive to stronger acid and base. U.V. irradiation causes conversion first to the 8,9-Z- isomer, then to unidentified decomposition products. Specific rotation [a]D22 +55.7?(c=0.87, CHCl3)
APPLICATIONS
Biochemistry Acts by stimulating the release of g-aminobutyric acid, an inhibitory neurotransmitter, thus causing paralysis. See M. J. Turner & J. M. Schaeffer in Ivermectin and Abamectin, W. C. Cambell ed., Springer-Verlag, New York (1989) p. 73. Mode of action Insecticide and acaricide with contact and stomach action. Has limited plant systemic activity, but exhibits translaminar movement. Uses Control of motile stages of mites, leaf miners, suckers, Colorado beetles, etc. on ornamentals, cotton, citrus fruit, pome fruit, nut crops, vegetables, potatoes, and other crops. Application rates are 5.6 to 28 g/ha for mite control, 11 to 22 g/ha for control of leaf miners. Also used for control of fire ants. Phytotoxicity May be phytotoxic to pome fruit when mixed with captan. Formulation types EC. Compatibility Not compatible with captan.
About price and product information, please click Message button.
Open the consulting page, please Writing information, customer manager will reply within 24 hours!
or Contact email:Donna.wen@molotuschem.com
Cell-phone number:17717500809
Tel:0086 21 61203246
Fax:0086 21 61612025
Address:Room 402, No.553, Maotai Road, Changning District, Shanghai.